DNAPLs are denser than water and have limited and varying solubilities in water; thus, they tend to occur in a separate nonaqueous phase in the subsurface. In contrast, alcohols (for example, ethanol) are fully soluble in water and do not form separate nonaqueous phase liquids (NAPLs). When DNAPLs are released to the subsurface, the DNAPL phase remains separate from the other principle fluid phases, air and water, and their subsurface migration and physical behavior are governed by the physics of multiphase flow in porous or fractured media.
This chapter describes the fluid properties of DNAPLs and their direct fluid-phase interaction with porous media. Chapter 3 discusses the evolving conceptual model for distribution and transport of DNAPL-forming compounds, which has necessitated an ongoing reevaluation of DNAPL site characterization objectives and methods.
Common DNAPL compounds include materials that have been or are still widely used in industrial and commercial processes, such as the following:
Possibly the most common DNAPLs are chlorinated solvents such as trichloroethylene (TCE), tetrachloroethylene (PCE), and carbon tetrachloride (CCl4). PCE and TCE have been used as degreasers, dry cleaning fluids, and solvents in many industrial and commercial processes, and have frequently been released to the subsurface. These solvents have also been commonly used in household products such as spot removers, brake cleaner, penetrating oils, typewriter correction fluid, and finishes.
Before 1985, carbon tetrachloride was used as a grain fumigant at almost every grain storage facility across the country; thus, soil and groundwater contamination with CCl4 and its primary degradation product chloroform is widespread across the Midwest and Plains areas of the United States (USEPA 1984). Also common is coal tar DNAPL contamination, resulting from historical spills/releases during manufactured gas plant (MGP) operations that produced coal gas for residential, commercial, and industrial uses. In portions of the country where wood treatment has been a major industry, creosote DNAPL contamination is also commonly encountered. Fuel oil products (such as No. 6 fuel oil DNAPL) may be encountered at industrial or government sites where they were used as boiler fuel, as well as at petroleum refineries and some electrical generating plants.
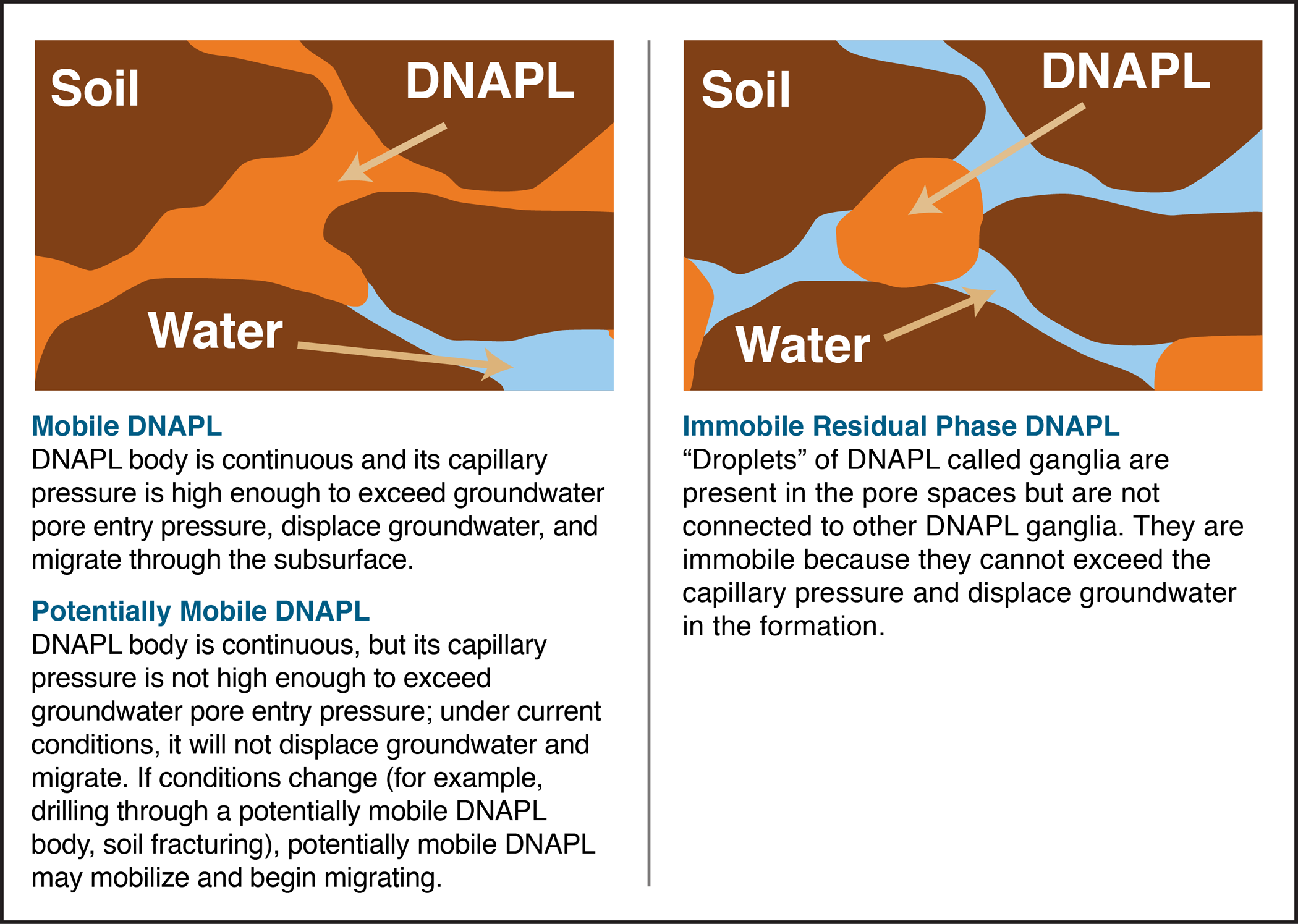
At sites where separate-phase DNAPL fluids are discovered in the subsurface, it is essential to recover and characterize fluid properties whenever possible. Each of the DNAPL fluid properties described in this section is important in helping assess the distribution and mobility of the separate-phase fluid or the partitioning/dissolution of compounds from the DNAPL to groundwater or soil vapor. Figure 2-1 describes the

Mobility characteristics of DNAPL: mobile, potentially mobile, and immobile.
Numerous references that provide fluid property data for neat (pure) liquids include Cohen et al. (1993), Pankow and Cherry (1996), Dwarakanath et al. (2002), and USEPA (1991); however, these data are generally not representative of DNAPL fluids after they have been released to the subsurface, and the errors that can be induced by relying on neat fluid characteristics to represent field conditions can be substantial. It is therefore important to measure site-specific DNAPL properties whenever possible rather than relying on literature-based values. DNAPL mixtures behave differently in the subsurface due to changes in the fluids’ characteristics, such as solubility, viscosity, and wettability. Table 2-1
Different types of DNAPLs have varying physical and chemical properties that govern their subsurface behavior. For example, chlorinated solvent DNAPLs have high solubility relative to other DNAPLs, and moderate solubility relative to the full spectrum of groundwater contaminants (see Figure 2-2). This can result in the development of significant dissolved-phase groundwater impacts. In contrast, No. 6 fuel oil has exceedingly low solubility and often does not result in significant dissolved-phase groundwater impacts; No. 6 fuel oil is also highly viscous, which limits its DNAPL migration in the subsurface. Furthermore, coal tar and creosote have been shown, in some circumstances, to behave in porous media as wetting fluids as opposed to nonwetting fluids (see
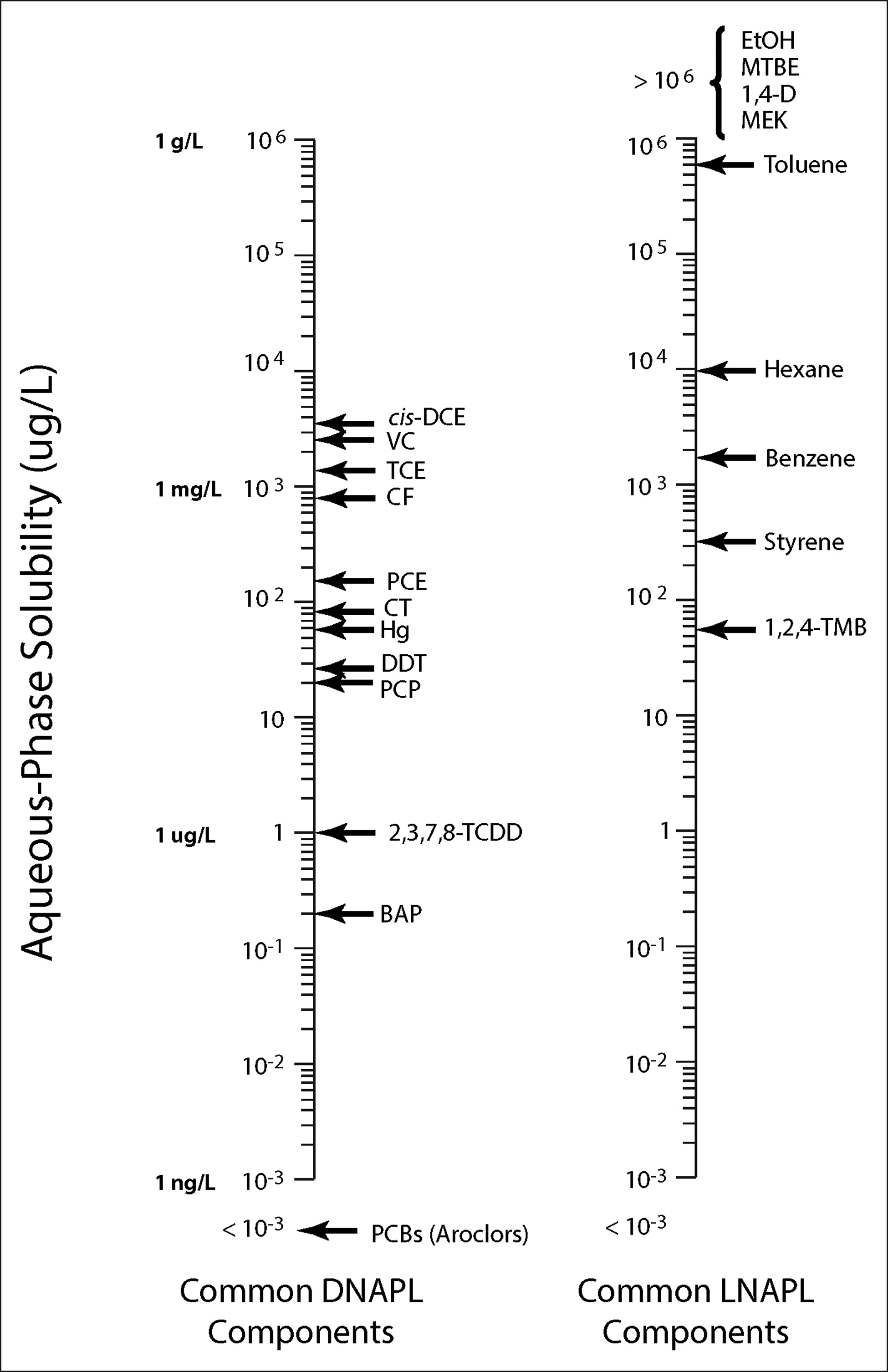
Solubilities of common DNAPLs and other compounds (see Appendix K for chemical acronym definitions).
Some of the actions that affect DNAPL in the porous media involve the combined properties of the fluid and its interaction with a particular subsurface media. As the subsurface geology creating the aquifer matrix is heterogeneous, interactions between the DNAPL and the subsurface may change over short distances with the changes in the aquifer matrix. Illustrating the combined matrix properties that represent conditions within a representative elementary volume (REV) helps to visualize the interactions between DNAPL and various portions of the aquifer matrix. Figure 2-3 illustrates that V1 and V2, both randomly selected to represent conditions of porosity of the whole mass, do not represent a REV of this site. V3, on the other hand, does appear to closely represent the REV of this site. An REV is the smallest subsurface element that can be considered to have homogeneous conditions representative of the system being evaluated.
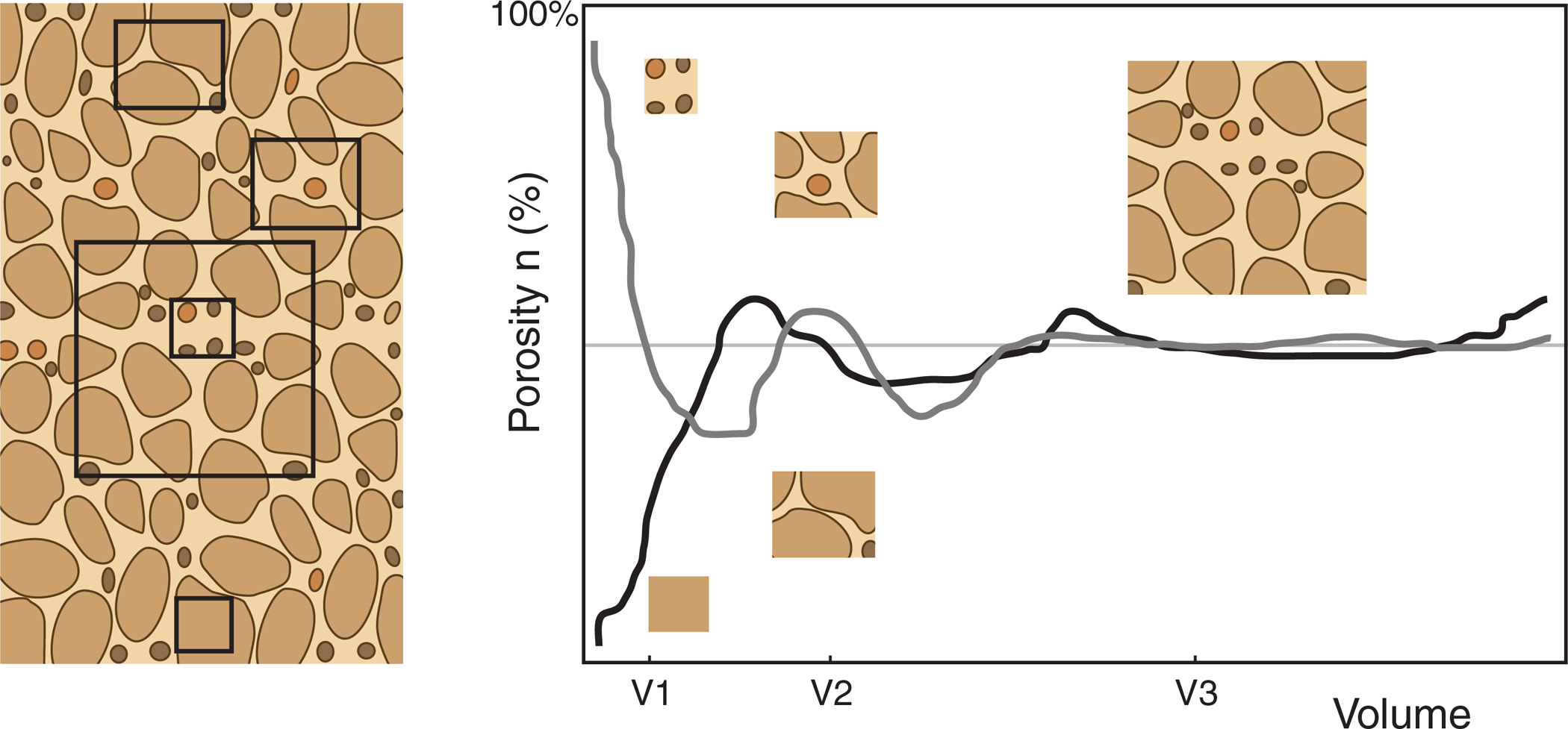
Representative elementary volume illustrated as a % porosity compared to increasing volumes of material. Beyond V3, the representativeness does not improve appreciably.
Source: S
Key DNAPL properties that relate to the site characterization concepts and tools described later in this guidance are listed below.
Aqueous solubility is a key DNAPL property, because it controls not only the ability of the DNAPL to produce and sustain a dissolved-phase plume, but also its longevity.
Density describes the mass per unit volume of the DNAPL. It is sometimes expressed as specific gravity (
Interfacial tension represents the force parallel to the interface of one fluid with another fluid (usually air or water), which leads to the formation of a meniscus and the development of capillary forces and a pressure difference between different fluids in the subsurface.
Viscosity (dynamic) represents the thickness or resistance to shear (flow) of the fluid. For example, honey is more viscous than water, which is more viscous than air.
Volatility represents the tendency of the DNAPL chemical constituents to evaporate into the vapor phase.
Wettability represents whether a fluid is wicked into or repelled out of the subsurface media, and is defined by the contact angle of the DNAPL fluid against the matrix materials in the presence of water. Wettability is a combined property of the DNAPL and the subsurface formation materials, and can be affected by chemistry and the presence of co-contaminants. Figure 2-4 illustrates the wetting process as an interaction between surfaces. The wetting process is an interaction between surfaces. In this example, the solid surface has sufficient force to overcome the surface tension on the low-surface tension droplet on the right and the droplet is stretched out into a thin wetting layer. The solid surface energy is not high enough to overcome the high surface tension of the droplet on the left and wetting does not occur
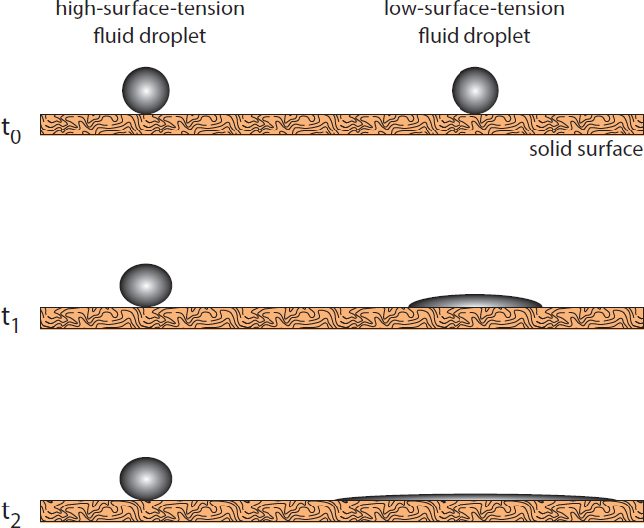
Illustration of the wetting process as an interaction between surfaces.
Saturation (
Residual saturation (
Relative permeability (
Capillary pressure (
Capillary entry pressure (
The importance and effects of the properties defined above on understanding DNAPL distribution, degradation, interactions with subsurface materials, and other behavior in the environment (as they apply to both characterizing contamination and developing remedial alternatives) are addressed in works by Cohen et al. (1993) and Pankow and Cherry (1996). Several of these key properties are summarized below; however, additional resources should be used, as needed, to assess the specific chemical and physical properties of the particular contaminant type(s) at a project site.
Aqueous solubility represents the maximum concentration of the DNAPL chemical constituents that can be dissolved in an aqueous solution (groundwater for the purpose of this document). For DNAPL mixtures, the aqueous solubility of individual components is subject to co-solvent effects. For example, under ideal conditions, the maximum concentration of a NAPL constituent (for example, PCE) is equal to the mole fraction of that constituent in the mixed NAPL multiplied by the aqueous solubility of the constituent if it were a pure NAPL (for example, PCE-DNAPL). Solubility considerations include the following:
Historically, a 1% dissolved-phase concentration of chlorinated solvent DNAPLs, based on compound-specific solubility in groundwater, was thought to indicate the potential presence of DNAPL; however, this method is now viewed as unreliable (that is, either falsely positive or falsely negative). See Appendix F for methods available to evaluate the presence of DNAPL (USEPA 2009).
Density/SG is a critical DNAPL property, as it affects the relative buoyancy of DNAPL in the saturated zone. While, by definition, all DNAPLs have an SG greater than 1.0, some DNAPLs (such as PCE) have an SG of >1.5, while others have an SG barely greater than water. For example, No. 6 fuel oil can sometimes exist as an LNAPL with an SG of <1.0 and in other cases may exist as a DNAPL with an SG of approximately 1.05. Denser DNAPLs have a greater driving force for downward movement, while other DNAPLs may be almost neutrally buoyant.
DNAPL Residual Saturation (DNAPL Sr), as described above, is a REV property and therefore represents conditions at a scale of centimeters that can vary greatly over small distances. A specific Sr at a site is a function of the DNAPL properties, including viscosity, interfacial tension, and wettability, and is particularly a function of the subsurface pore structure. Residual saturation represents the saturation at which the DNAPL is immobilized by capillary forces as discontinuous ganglia under ambient groundwater flow conditions (Cohen et al. 1993). Residual saturation can be affected by conditions such as temperature and groundwater chemistry, which can influence viscosity or interfacial tension. Residual saturation is also affected by groundwater gradients. Increased groundwater gradients (such as from active remediation) can mobilize residual DNAPL effectively by decreasing the residual saturation value. Similar to permeability or porosity, Sr is a REV property and can vary greatly between immediately adjacent geologic materials. The ultimate distribution of residual DNAPL is not uniform or readily predictable in the subsurface due to minute variations in pore size distributions, soil texture, soil structure, and mineralogy. DNAPL Sr typically ranges from 5% to 15%, and site-specific measurement of Sr can be potentially valuable.
Interfacial tension, viscosity, fluid density, and porous media characteristics are properties that govern DNAPL migration. In the typical scenario, water is the wicking or wetting fluid (see Figure 2-5) and DNAPL is non-wetting; however, for some aquifer matrices and some DNAPLs, the system may be DNAPL-wetting. Also, in a low-moisture vadose zone setting above the water table, even non-wetting DNAPL can be subject to capillary spreading, as DNAPL often acts as a wetting fluid relative to air. Below the water table, most but not all DNAPLs behave as non-wetting fluids. In these cases, the interfacial tension between the DNAPL and water phases act to resist DNAPL spreading (see Figure 2-6). This resistance to DNAPL movement is expressed as a pressure difference between the DNAPL and water phases, referred to as Pc. Furthermore, there are differences in the subsurface pore structure, which control the Pc that resists DNAPL movement. Therefore, subsurface geologic heterogeneity ultimately controls DNAPL migration.
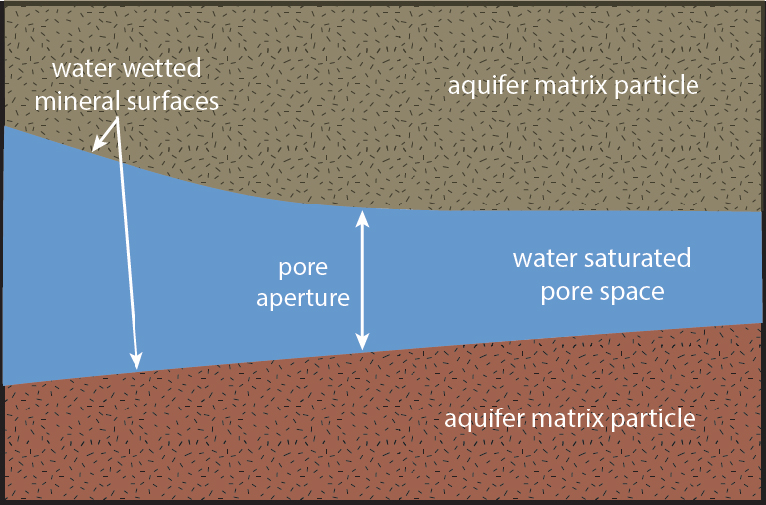
Typical conditions found in an aquifer with water present.
Figure 2-6 depicts the invasion of a NAPL into a saturated porous medium. The pore aperture is narrowing, from the left to the right. As the pore aperture narrows, the capillary pressure of the wetting fluid (water, in this case) increases, which increases the NAPL entry pressure, Pe. On the left, the NAPL pool pressure exceeds the entry pressure and the free water phase has been displaced, leaving only the wetting layer on the mineral surface. In the middle, the NAPL pool pressure matches the capillary pressure of the water, and because the two forces are in balance, the NAPL invasion has stopped. To the right, the pore aperture is smaller and the capillary pressure of the wetting fluid exceeds the NAPL pressure. As a result, the NAPL cannot invade this portion of the pore, without a change in the fluid conditions. Changes that could permit further invasion include (1) increased NAPL pool height, which increases the NAPL pressure; (2) decreasing interfacial tension, which can occur as a result of biodegradation of dissolved-phase NAPL; and (3) groundwater extraction from beneath the NAPL, which increases the effective NAPL pool pressure.
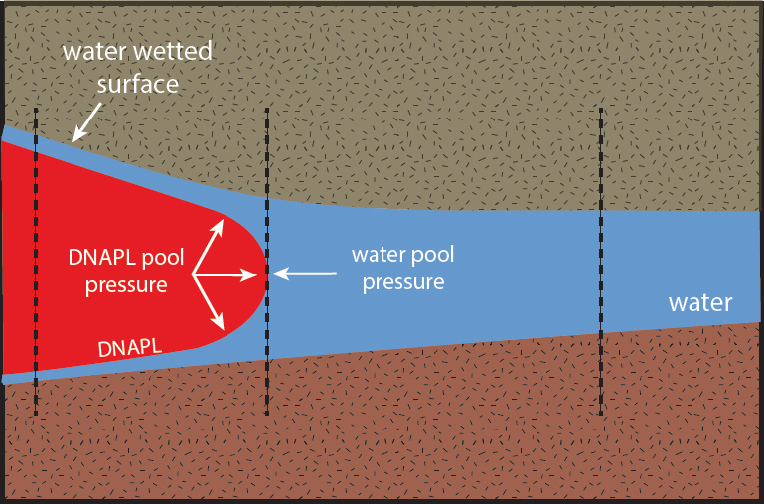
Typical conditions in an aquifer with DNAPL present when water is the wetting fluid.
S, Pc, and kr are interrelated and complex properties that govern fluid movement when multiple fluids are present within pore space in a REV, such as when DNAPL is present below the water table. Pc and kr are both nonlinear functions of S, and DNAPL mobility is governed by the nonlinear S-Pc-kr constitutive relationships. At most sites, characterization of the nonlinear constitutive relationships is not necessary unless multiphase flow modeling is undertaken; however, some related concepts are important in guiding the site characterization activities described in this document.
As described above, at Sr, DNAPL is immobile. Similarly, at very low S, approaching the value of Sr, DNAPL mobility is limited because kr is very small. Increasing DNAPL mobility (increasing kr) can be realized most often through changes in pressure conditions affecting Pc or by changes in chemistry that affect interfacial tension. As described above, the Pce is the minimum pressure that must be overcome for DNAPL to displace water and advance its migration into new geologic materials. Subsurface geologic materials with smaller pore structure (finer grained or more densely packed) have higher Pces; therefore, DNAPL migration tends to follow the largest, most accessible pore structures. In fine-grained sediments or consolidated rock, large pore structures such as fractures or other secondary porosity pathways (such as root holes and dissolution features) may be available for migration.
DNAPL viscosity affects the rate at which DNAPL can migrate through the subsurface, but does not inherently influence whether or not DNAPL can migrate. DNAPL viscosity primarily comes into play if there are mobile and potentially recoverable volumes of DNAPL, as highly viscous DNAPLs such as No. 6 fuel oil, creosote, and coal tar are slow to migrate.
The volatility of DNAPL constituents, as measured by vapor pressure, directly affects partitioning or transfer of these constituents into the vapor phase, either directly from a DNAPL that may be present in the vadose zone or from dissolved DNAPL constituents in groundwater. Mixed DNAPLs have reduced component vapor pressures, which can be estimated from pure compound vapor pressure data and by measuring the DNAPL solution composition.
The vapor pressure is the pressure exerted by the vapor phase of a substance at equilibrium with the pure condensed (solid or liquid) phase in a closed system. For ideal gases, the vapor pressure can be converted to a concentration using the ideal gas law at a given temperature and pressure. Vapor pressure data are used in predicting the equilibrium partitioning constants between the gas phase and liquid or solid phases.
For dilute solutions, Henry’s law is the ratio of the vapor pressure to the solubility of a substance; that is, solubility and vapor pressure are directly proportional, at a given temperature and pressure, for sparingly soluble compounds. Using the ideal gas constant and temperature, the Henry’s law constant can be converted to a dimensionless form that is the ratio of concentration in air to the concentration in water at equilibrium at a given temperature and pressure. Use of the Henry’s law constant assumes a linear relationship between the partial pressure of a substance and its concentration in water up to saturation, which is generally only true in dilute solutions.
Where KH is Henry’s law constant, PA is the partial pressure, Awater is the concentration of a substance in water, Kaw is the dimensionless Henry’s law constant, and Aair is the concentration of a substance in air.
For an ideal solution, Raoult’s law states that the mole fraction of each substance in a mixture is equal to the mole fraction of the vapor pressure of that substance in a closed syst
Where Pi is the partial pressure of substance i, xi is the mole fraction of substance i in the mixture, Pio is the vapor pressure of i, ni is the number of moles of i, and j is the number of substances in the mixture. Raoult’s law is generally used for mixtures of organic compounds, such as petroleum or solvents.
Fick’s first law states that flux is directly related to the concentration gradient of a chemical multiplied by a diffusion coefficient for that chemical. Diffusion can only be affected by the manipulation of one of these terms, which limits remedial alternatives where diffusion controls mass transfer.
The Peclet number is the ratio of advective transport rate to diffusive transport rate. It is used to estimate where diffusive transport or advective transport dominate under a given set of conditions. In low Peclet number environments, Fick’s first law predominates. In high Peclet number environments, the fluid velocity controls transport.
Where Pe is the Peclet number, L is the characteristic length, U is the velocity, and D is the mass diffusion coefficient for a given chemical.
DNAPL mixtures such as creosote and MGP tars, which are characterized by high viscosity and low solubility, generally have a higher potential to migrate in the subsurface, and for longer periods, than other DNAPL types such as chlorinated solvents. The relatively high viscosity of these DNAPL mixtures leads to longer-term continuity of the mobile DNAPL phase, sustaining mobility and contributing to mobility within small-scale geologic features. The relatively low solubility of many of the components of these DNAPL mixtures leads to less weathering of the DNAPL mixture in the environment, maintaining the mobile DNAPL phase longer than DNAPL types that contains higher solubility components. Finally, DNAPL mixtures that contain large fractions of heavy-weight hydrocarbons have been shown to alter the solid phase wettability that can also sustain DNAPL mobility. For example, prolonged contact of MGP tar with soil can cause water-wet soil to effectively become DNAPL-wet. When a soil becomes DNAPL-wet, the residual saturation decreases, resulting in lower DNAPL retention within the soil structure, sustaining the DNAPL migration potential of DNAPL mixtures for longer periods of time as compared to pure component DNAPL releases.
The DNAPL may also have weathered over time in the subsurface since its release. Some industrial-grade DNAPLs have impurities that, while acceptable to a manufacturing process, change the properties enough to influence subsurface fate and transport in a manner different from those of the pure DNAPLs. Therefore, at any DNAPL site, analysis of site-specific NAPL acquired from the subsurface is recommended if at all possible to facilitate site assessment. In addition to the potentially significant differences between pure and aged NAPLs, properties of similar NAPLs at different sites and in different site areas can also vary.
Physical properties (density, viscosity, and interfacial tension) of water, select reference fluids, and field aged/weathered DNAPLs—including chlorinated solvents, mixed DNAPLs, MGP coal tar, and coal tar creosote—are presented in Table 2-1. The published literature and the measurements contained in Table 2-1 indicate the following:
Coal tar and creosote DNAPL is also encountered below ground at many former MGP and wood-treating sites where substantial releases occurred over decades. The large volume, low solubility, and high viscosity of coal tar and DNAPL released at wood-treating and former MGP sites can result in much longer periods (often decades) of DNAPL migration than typically occurs at chlorinated solvent release sites. Measured physical property values from former MGP sites in the Unites States typically range from 1.02 to 1.10 g/cc density, 20 to 100 cP viscosity, and 15 to 27 dynes/cm interfacial tension.